App Controlled Knee Pain Relief Device No Longer Requires Prescription
Motive Health Inc. announced the Motive Knee.
MotiveTM Knee
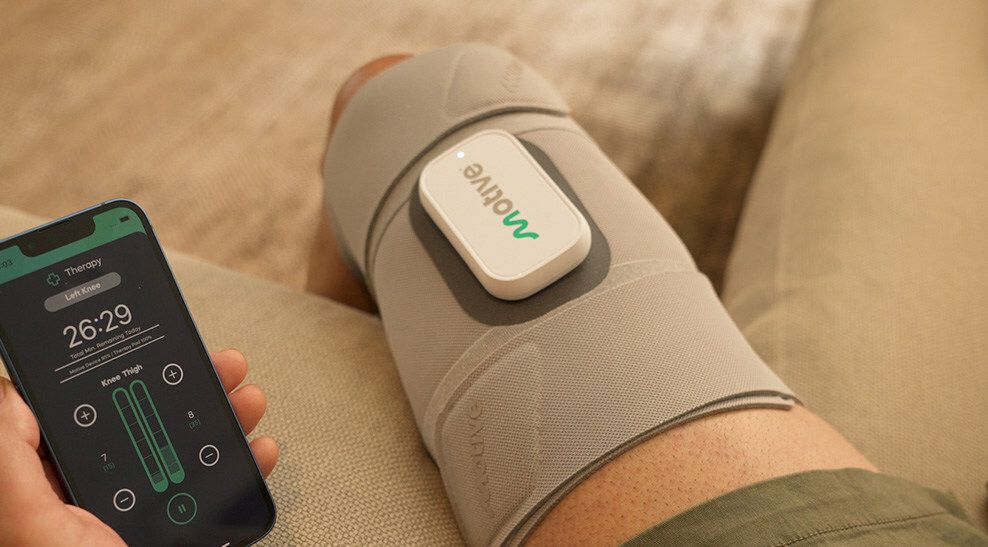
Motive Health Inc. announced in a press release that the Motive Knee, a pain relief device, will not require a prescription. The FDA-approved device had previously required one.
The device, which patients can use at home, pairs with a mobile app that controls therapy levels, monitors progress, and charts mobility goals. The device uses precise electrical pulses to stimulate muscles near the knee, which is intended to promote growth in the quadriceps. Motive Health recommends using it for 30 minutes a day to relieve pain.
In a press release, president and CEO of Motive Health Rob Morocco said, “The direct-to-consumer launch of Motive Health is an exciting time not only for the company but for consumers who can now have access to lasting knee pain relief from home. Our technology is a game changer for anyone trying to get back to an active lifestyle that is limited by debilitating knee pain. We are delighted to make Motive Knee accessible to those in need through our new e-commerce platform."
According to Motive Health, allowing the MotiveTM Knee to be sold without a prescription will allow the company to provide it a wider audience.